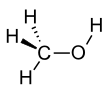
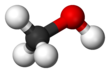
മെഥനോൾ
രാസ സംയുക്തം
(Methanol എന്ന താളിൽ നിന്നും തിരിച്ചുവിട്ടതു പ്രകാരം)
CH3OH എന്ന തന്മാത്രാ ഘടനയുള്ള രാസവസ്തുവാണ് മെഥനോൾ, ( മീഥൈൽ ആൽക്കഹോൾ / വുഡ് ആൽക്കഹോൾ / വുഡ് നാഫ്ത / വൂഡ് സ്പിരിറ്റ്) [17]
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Methanol[1] | |||
| Other names
Carbinol
Columbian spirits Hydroxymethane MeOH Methyl alcohol Methyl hydrate Methyl hydroxide Methylic alcohol Methylol Methylene hydrate, primary alcohol Pyroligneous spirit Wood alcohol Wood naphtha Wood spirit | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| Beilstein Reference | 1098229 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.599 | ||
| EC Number |
| ||
| Gmelin Reference | 449 | ||
| KEGG | |||
| MeSH | {{{value}}} | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1230 | ||
CompTox Dashboard (EPA)
|
|||
| InChI | |||
| SMILES | |||
| Properties | |||
| തന്മാത്രാ വാക്യം | |||
| Molar mass | 0 g mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | Faint and similar to ethanol | ||
| സാന്ദ്രത | 0.792 g/cm3[2] | ||
| ദ്രവണാങ്കം | |||
| ക്വഥനാങ്കം | |||
| miscible | |||
| log P | −0.69 | ||
| ബാഷ്പമർദ്ദം | 13.02 kPa (at 20 °C) | ||
| അമ്ലത്വം (pKa) | 15.5[3] | ||
| Conjugate acid | Methyloxonium[4] | ||
| Conjugate base | Methanolate[5] | ||
| −21.40·10−6 cm3/mol | |||
| Refractive index (nD) | 1.33141[6] | ||
| വിസ്കോസിറ്റി | 0.545 mPa·s (at 25 °C)[7] | ||
| 1.69 D | |||
| Hazards[12][13] | |||
| Main hazards | Methanol and its vapours are flammable.
Moderately toxic for small animals – Highly toxic to large animals and humans (in high concentrations) – May be fatal/lethal or cause blindness and damage to the liver, kidneys, and heart if swallowed – Toxicity effects from repeated over exposure have an accumulative effect on the central nervous system, especially the optic nerve – Symptoms may be delayed, become severe after 12 to 18 hours, and linger for several days after exposure[9] | ||
| Safety data sheet | [1] | ||
| GHS pictograms |    [8] [8]
| ||
| GHS Signal word | Danger[8] | ||
| H225, H301, H302, H305, H311, H331, H370[8] | |||
| P210, P233, P240, P241, P242, P243, P260, P264, P270, P271, P280, P301+330+331, P310, P302+352, P312, P303+361+353, P304+340, P311, P305+351+338, P307+311, P337+313, P361, P363, P370+378, P403+233[8] | |||
| Flash point | {{{value}}} | ||
| Explosive limits | 6–36%[10] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
5628 mg/kg (rat, oral) 7300 mg/kg (mouse, oral) 12880 mg/kg (rat, oral) 14200 mg/kg (rabbit, oral)[11] | ||
LC50 (median concentration)
|
64,000 ppm (rat, 4 h)[11] | ||
LCLo (lowest published)
|
33,082 ppm (cat, 6 h) 37,594 ppm (mouse, 2 h)[11] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 200 ppm (260 mg/m3)[10] | ||
REL (Recommended)
|
TWA 200 ppm (260 mg/m3) ST 250 ppm (325 mg/m3) [skin][10] | ||
IDLH (Immediate danger)
|
6000 ppm[10] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
അവലംബം
തിരുത്തുക- ↑ Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 692. doi:10.1039/9781849733069-00648. ISBN 978-0-85404-182-4.
- ↑ Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ↑ Ballinger, P.; Long, F. A. (1960). "Acid Ionization Constants of Alcohols. II. Acidities of Some Substituted Methanols and Related Compounds". J. Am. Chem. Soc. 82 (4): 795–798. doi:10.1021/ja01489a008.
- ↑ "Methyloxonium". pubchem.ncbi.nlm.nih.gov. Archived from the original on 21 ഡിസംബർ 2018. Retrieved 21 ഡിസംബർ 2018.
- ↑ "Methanolate". pubchem.ncbi.nlm.nih.gov. Archived from the original on 21 ഡിസംബർ 2018. Retrieved 21 ഡിസംബർ 2018.
Methoxide is an organic anion that is the conjugate base of methanol. ... It is a conjugate base of a methanol.
- ↑ "RefractiveIndex.INFO – Refractive index database". refractiveindex.info. Archived from the original on 23 ഫെബ്രുവരി 2017. Retrieved 14 ഫെബ്രുവരി 2012.
- ↑ González, Begoña (2007). "Density, dynamic viscosity, and derived properties of binary mixtures of methanol or ethanol with water, ethyl acetate, and methyl acetate at T = (293.15, 298.15, and 303.15) K". The Journal of Chemical Thermodynamics. 39 (12): 1578–1588. Bibcode:2007JChTh..39.1578G. doi:10.1016/j.jct.2007.05.004.
- ↑ 8.0 8.1 8.2 8.3 "Methanol" (PDF). Lab Chem. Valtech. Archived (PDF) from the original on 10 മാർച്ച് 2016. Retrieved 10 മാർച്ച് 2016.
- ↑ Toxicity on PubChem Archived 20 August 2018 at the Wayback Machine.
- ↑ 10.0 10.1 10.2 10.3 "NIOSH Pocket Guide to Chemical Hazards #0397". National Institute for Occupational Safety and Health (NIOSH).
- ↑ 11.0 11.1 11.2 "Methanol". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- ↑ 12.0 12.1 "The Emergency Response Safety and Health Database: Systematic Agent: METHANOL". Centers for Disease Control and Prevention. Archived from the original on 23 ഏപ്രിൽ 2009. Retrieved 3 ഓഗസ്റ്റ് 2018.
- ↑ "PubChem: Safety and Hazards - GHS Classification". National Center for Biotechnology Information, U.S. National Library of Medicine. Archived from the original on 20 ഓഗസ്റ്റ് 2018. Retrieved 20 ഓഗസ്റ്റ് 2018.
- ↑ "Methanol Safe Handling Manual" (PDF). Methanol Institute. 2017. p. 253. Archived (PDF) from the original on 20 ഡിസംബർ 2017. Retrieved 3 ഓഗസ്റ്റ് 2018.
- ↑ "Technical Information & Safe Handling Guide for Methanol". Methanex Corporation. Archived from the original on 11 മാർച്ച് 2012.
- ↑ "Methanol Safe Handling Manual" (PDF). Methanol Institute. 2017. p. 243. Archived (PDF) from the original on 20 ഡിസംബർ 2017. Retrieved 3 ഓഗസ്റ്റ് 2018.
- ↑ https://en.wikipedia.org/wiki/Methanol